Effect of the Fermented Soy Q-CAN® Product on Biomarkers of Inflammation and Oxidation in Adults with Cardiovascular Risk, and Canonical Correlations between the Inflammation Biomarkers and Blood Lipids
2023-08-16
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10383246/
https://www.mdpi.com/2072-6643/15/14/3195
Effect of the Fermented Soy Q-CAN® Product on Biomarkers of
Inflammation and Oxidation in Adults with Cardiovascular
Risk, and Canonical Correlations between the Inflammation
Biomarkers and Blood Lipids
Sarah M. Jung 1,2, Amandeep Kaur 1, Rita I. Amen 1, Keiji Oda 1, Sujatha Rajaram 1, Joan Sabatè 1
and Ella H. Haddad 1,*
1 Center for Nutrition, Healthy Lifestyle and Disease Prevention, Loma Linda University,
Loma Linda, CA 92350, USA; sjung15@calstatela.edu (S.M.J.); akaur1@llu.edu (A.K.);
ramen@students.llu.edu (R.I.A.)
2 Rongxiang Xu College of Health and Human Services, California State University Los Angeles,
Los Angeles, CA 90032, USA
* Correspondence: ehaddad@llu.edu
Abstract: Systemic low-grade inflammation plays a key role in the development of cardiovascular
disease (CVD) but the process may be modulated by consuming fermented soy foods. Here, we
aim to evaluate the effect of a fermented soy powder Q-CAN® on inflammatory and oxidation
biomarkers in subjects with cardiovascular risk. In a randomized crossover trial, 27 adults (mean
age ± SD, 51.6 ± 13.5 y) with a mean BMI ± SD of 32.3 ± 7.3 kg/m2 consumed 25 g daily of the
fermented soy powder or an isoenergic control powder of sprouted brown rice for 12 weeks each.
Between-treatment results showed a 12% increase in interleukin-1 receptor agonist (IL-1Ra) in the
treatment group, whereas within-treatment results showed 23% and 7% increases in interleukin-6
(IL-6) and total antioxidant status (TAS), respectively. The first canonical correlation coefficient
(r = 0.72) between inflammation markers and blood lipids indicated a positive association between
high-sensitivity C-reactive protein (hsCRP) and IL-1Ra with LDL-C and a negative association with
HDL-C that explained 62% of the variability in the biomarkers. These outcomes suggest that blood
lipids and inflammatory markers are highly correlated and that ingestion of the fermented soy
powder Q-CAN® may increase IL-1Ra, IL-6, and TAS in individuals with CVD risk factors.
 interleukin-6 (IL-6), and soluble tumor necrosis factor (TNF) receptors in middle-aged
interleukin-6 (IL-6), and soluble tumor necrosis factor (TNF) receptors in middle-aged
Chinese women [10] and data from the National Health and Nutrition Examination Survey
(NHANES) 2005–2008 showed an association between lower circulating C-reactive protein
(CRP) concentrations and higher urine excretion of isoflavonoids [11].
Subsequently, multiple human clinical trials tested various types, schedules, and doses
of dietary soy or soy isoflavone-rich foods and supplements. Given the heterogeneity
in population and design, these studies produced interesting but divergent results. Soy
products inhibited inflammatory markers in postmenopausal women [12–14], in women
with metabolic syndrome [15], along with exercise in overweight women [16] or along with
vitamin D in individuals with irritable bowel [17], in those with non-alcoholic fatty liver
disease [18] and men with prostate cancer [19]. Alternatively, other studies reported no
anti-inflammatory effects in healthy postmenopausal women [20–22], and in those with
mild hypercholesterolemia [23]. Although some soy or isoflavone interventions may not
have shown outcomes on inflammation markers, other cardiovascular benefits related to
immune and endothelial function were noted [24–26].
Many traditional soy foods such as tofu, tempeh, miso, and natto are fermented
products that require the involvement of microorganisms or fungi to produce. Microbial
fermentation elaborates hydrolytic protease and β-glucosidase enzymes that produce
bioactive peptides and convert glycosidic isoflavone glycosides into aglycones which
improve the digestibility of soybeans, increasing the quantity and bioavailability of its
bioactive principles, and may exert anti-inflammatory effects [27,28]. In our previous
report on this clinical trial, we demonstrated that the consumption of the fermented soy
powder marketed as Q-CAN® Natural lowered total- and LDL-C in adult participants
at cardiovascular risk [29]. In the current analysis, we explore our secondary objective
which was to determine the impact of the fermented soy supplement on biomarkers of
inflammation and oxidation. Also, canonical correlation analysis will be used to examine
correlations between inflammatory and antioxidant biomarkers and blood lipids.
2. Materials and Methods
2.1. Study Design and Participants
A detailed description of the study design, procedures, participant recruitment, and
randomization was previously described and published [29]. Briefly, the present study
was conducted as a randomized placebo-controlled crossover dietary food product intervention performed in two 12-week periods with a 2-week washout between interventions. Study participants were adult men and women aged 29–75 years with at least
2 risk factors for CVD. The following were considered CVD risk factors: overweight
and obesity with BMI kg/m2 ≥ 25; elevated systolic blood pressure ≥ 140/90 mmHg,
LDL-C ≥ 110 mg/dL, triglycerides ≥ 150 mg/dL, and fasting blood glucose ≥ 110 mg/dL;
low HDL-C ≤ 40 mg/dL, smoking and family history of heart disease. Exclusion criteria
were any renal, hepatic, or endocrine conditions that were not being managed, allergy
or sensitivity to soy or brown rice, alcohol or drug abuse, type 1 diabetes, and inability
to adhere to the study protocol. Also excluded were those who were pregnant, likely to
become pregnant, or lactating during the study period.
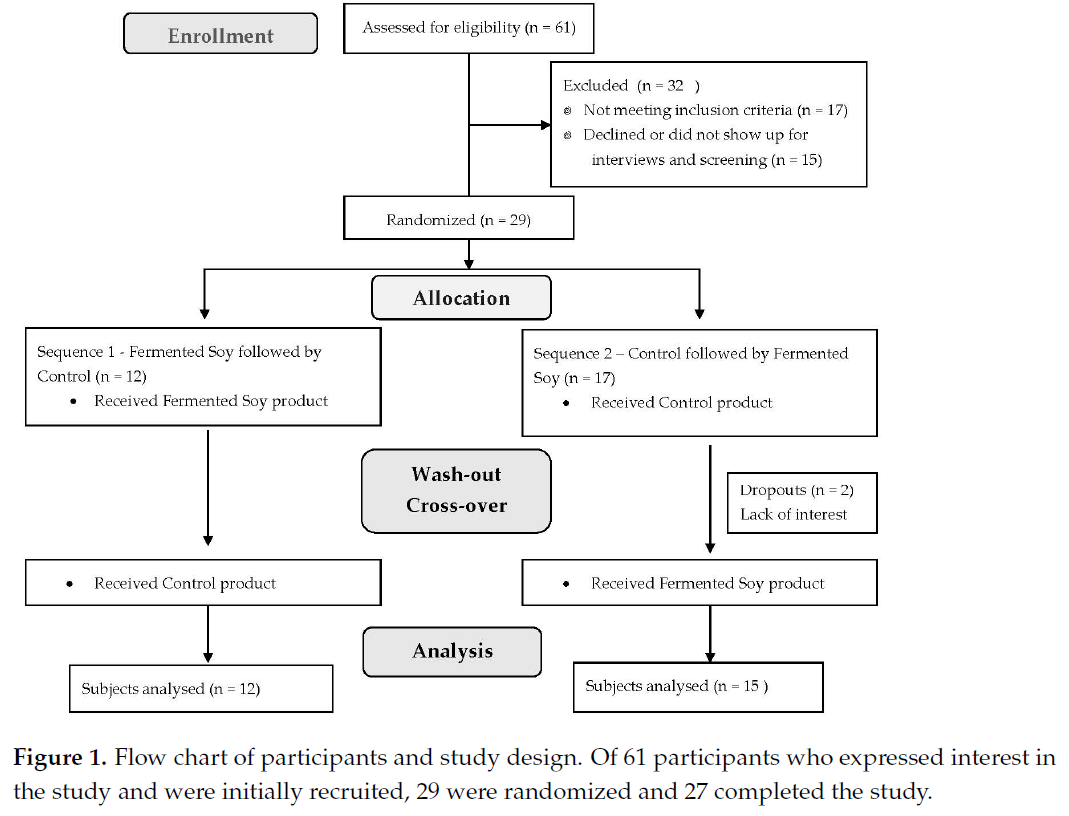
The study included 27 participants in free-living conditions who were randomly
assigned to 2 study sequences, either fermented soy powder followed by the control
brown rice powder or vice versa in a typical crossover fashion. Participants were enrolled
at 3 separate time points. Participants were instructed to maintain their usual physical
activity levels and consistent lifestyle habits during the study period. Of 61 subjects
screened, 29 were randomized and 2 participants dropped out for lack of interest. A flow
chart of participants is shown in Figure 1.
The study was conducted at the Nutrition Research Center of Loma Linda University
(LLU) in Loma Linda, CA. The study protocol and relevant documents were approved by
the Institutional Review Board of Loma Linda University before the initiation of the trial,
and participants provided written informed consent before enrolling in the study. This clinical trial was conducted according to the ethical study guidelines of the Declaration
of Helsinki of 1975 as revised in 1983. The study was registered at ClinicalTrials.gov as
NCT03429920.
2.2. Study Dietary Supplements

 dardized calibrated scale. Height was measured within 0.10 cm using a wall-mounted
dardized calibrated scale. Height was measured within 0.10 cm using a wall-mounted
stadiometer. Body fat was measured using the TANITA bio-impedance analyzer (TBF 305)
and waist circumference was taken with a non-distensible tape measure using a standard
mercury sphygmomanometer.
After an overnight fast (≥10 h), blood samples were collected at baseline and the end
of each 12-week period of the trial. After separation by centrifugation, serum, and plasma
were aliquoted and stored immediately at -80 ◦C until the project was completed. Blood
lipids were analyzed at the Analytical Core Laboratory, John Mayer USDA Human Research
Center at Tufts University (Medford, NJ, USA) on an automated AU480 Clinical Chemistry
Analyzer (Beckman Coulter, Inc., Brea, CA, USA), as specified in the manufacturer’s procedural documentation, and high sensitivity C-reactive protein (hsCRP) was measured by
solid-phase, two-site chemiluminescent immunometric assays using the IMMULITE 2000,
(Siemens Healthcare Diagnostics, Los Angeles, CA, USA). Tests per subject were conducted
in the same analytical run to reduce systematic error and inter-assay variability. Inflammation and oxidation markers were assayed at the LLU nutrition laboratory using commercial
ELISA and colorimetric kits. Tests for plasma human (IL-1), interleukin-1 receptor inhibitor
(IL-1Ra), TNF-α, haptoglobin, and thiobarbituric acid reactive substances (TBARS) were assayed using ELISA kits obtained from R&D Systems (Minneapolis, MN, USA). The human
IL-6 was from Abcam (Cambridge, MA, USA) and the colorimetric kit used to assay total
antioxidant status (TAS) was from Millipore Sigma (Burlington, MA, USA). All samples
were assayed in duplicate according to the manufacturer’s directions and absorbance was
measured on an automatic microplate reader (Bio Tek Synergy HT, Winooski, VT, USA).
Intra- and inter-assay CV were <10% on all assays.
2.4. Statistical Analysis
As previously reported [29], the study sample size was calculated considering the
primary objective of the intervention which was changes in LDL-C. Between-group differences in participant characteristics at baseline were assessed by two-sample t-tests or
Fisher’s exact tests and reported as means and standard deviations (SD) or assessed by
Mann–Whitney tests and reported as medians and interquartile ranges (IQR). All inflammatory and oxidation variables were log-transformed prior to analysis to normalize their
distribution and back-transformed to the original scale as geometric means. For each of the
outcomes, a mixed model was fitted to compare changes from baseline to end of treatment
and between treatments. The mixed models included treatment (fermented soy, brown
rice), time (baseline, end), the interaction between treatment and time, sequence (1, 2),
period (first, second), and enrollment set (1, 2, 3) as fixed effects terms and subjects as a
random-effects term. A difference in log means was also back-transformed and expressed
as a mean ratio between the two treatments.
To examine if the effect of treatment was different between sexes (male, female), a
3-way interaction of treatment × time × sex was applied. Among all markers, the 3-way
interaction was significant for TNF-α and TAS which were included in the stratified analysis.
Similarly, to examine if the effect of treatment was different between obese (BMI ≥ 30) and
non-obese (BMI < 30) at baseline, a dichotomous BMI variable was created and added into
the original model for a 3-way interaction of treatment x time x BMI. The 3-way interaction
was significant for haptoglobin which was included in the BMI-stratified analysis.
Canonical correlation analysis was used to explore the inter-relationship between
blood lipids and inflammatory and oxidation markers. Partial correlation coefficients were
first computed between the lipid markers, the inflammatory markers, and between the
lipid and inflammatory markers and adjusted for age and sex to exclude associations
that were highly correlated. Canonical variates 1 and 2 were then created for lipids
and the inflammatory markers and the 1st and 2nd canonical correlations between the
two were computed.
Unless stated otherwise, significance tests were 2-sided and were performed at a
5% level of significance. All statistical analyses were conducted with the use of SAS
software (version 9.4: SAS Institute Inc., Cary, NC, USA) and R version 3.6.3.
3. Results
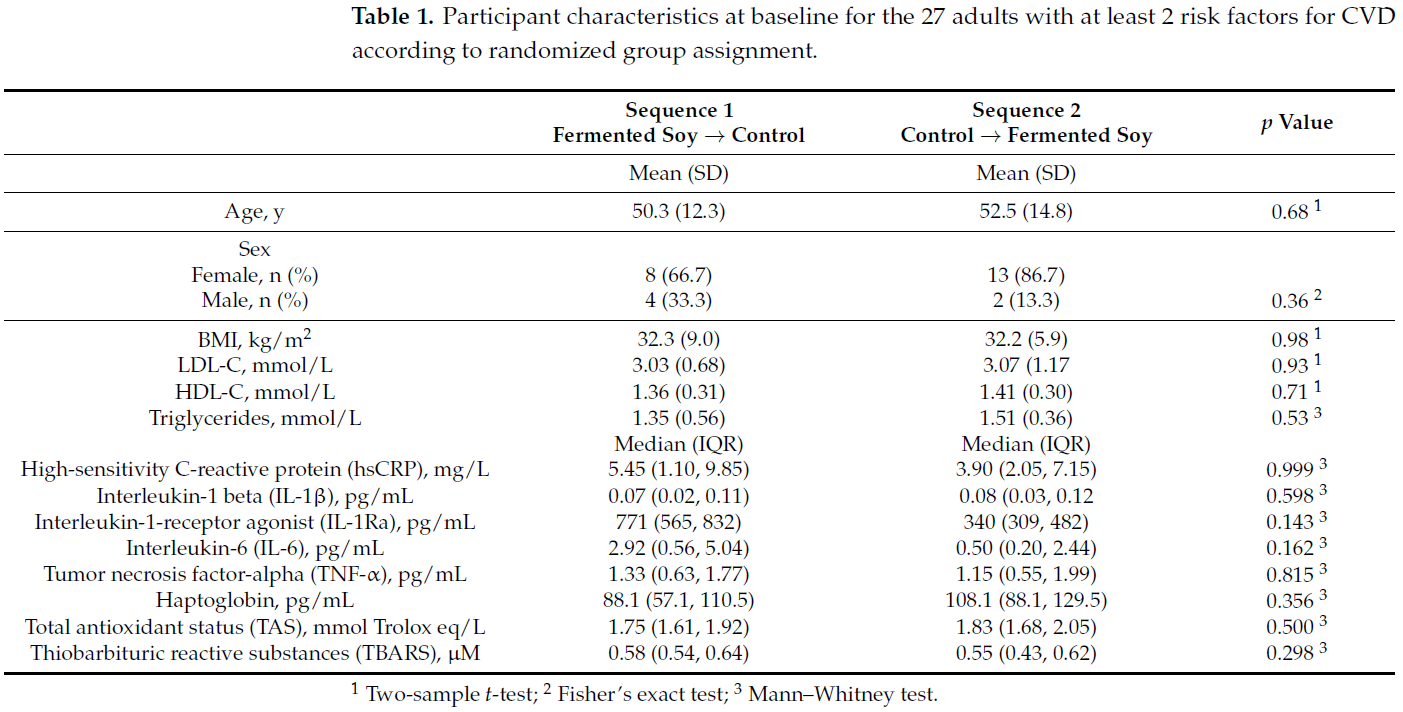
3.1. Sample Characteristics
Of the 29 randomized participants, 21 females and 6 males completed the study and
2 dropped out due to lack of interest. The baseline characteristics were similar between
groups as shown in Table 1.
Table 1. Participant characteristics at baseline for the 27 adults with at least 2 risk factors for CVD
according to randomized group assignment.
 3.2. Effect of Fermented Soy Supplementation on Inflammation and Oxidation Markers
3.2. Effect of Fermented Soy Supplementation on Inflammation and Oxidation Markers
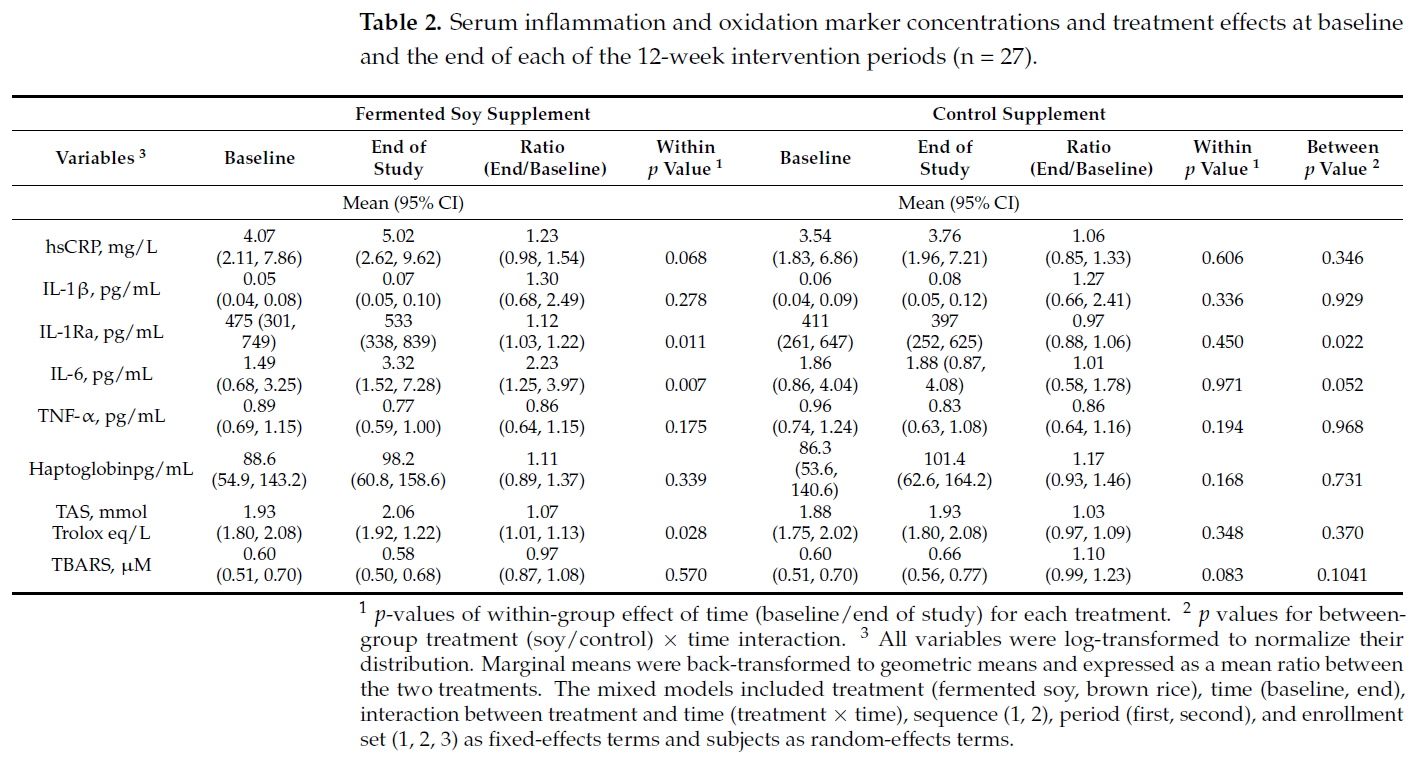
Values for inflammatory and oxidation biomarkers at baseline and end of each intervention are shown in Table 2. After 12 weeks, there was a significant increase in withintreatment group levels of IL-1Ra, IL-6 and TAS in those on the fermented soy supplement
and no change in any of the markers in those on the control brown rice supplement. However, between-group analysis only showed a significant effect for IL-1Ra, whereas IL-6
showed a tendency towards significance. There were no significant between-group differences in hsCRP, IL-1, TNF-α, haptoglobin, TAS or TBARS in comparing the active and
control treatments.
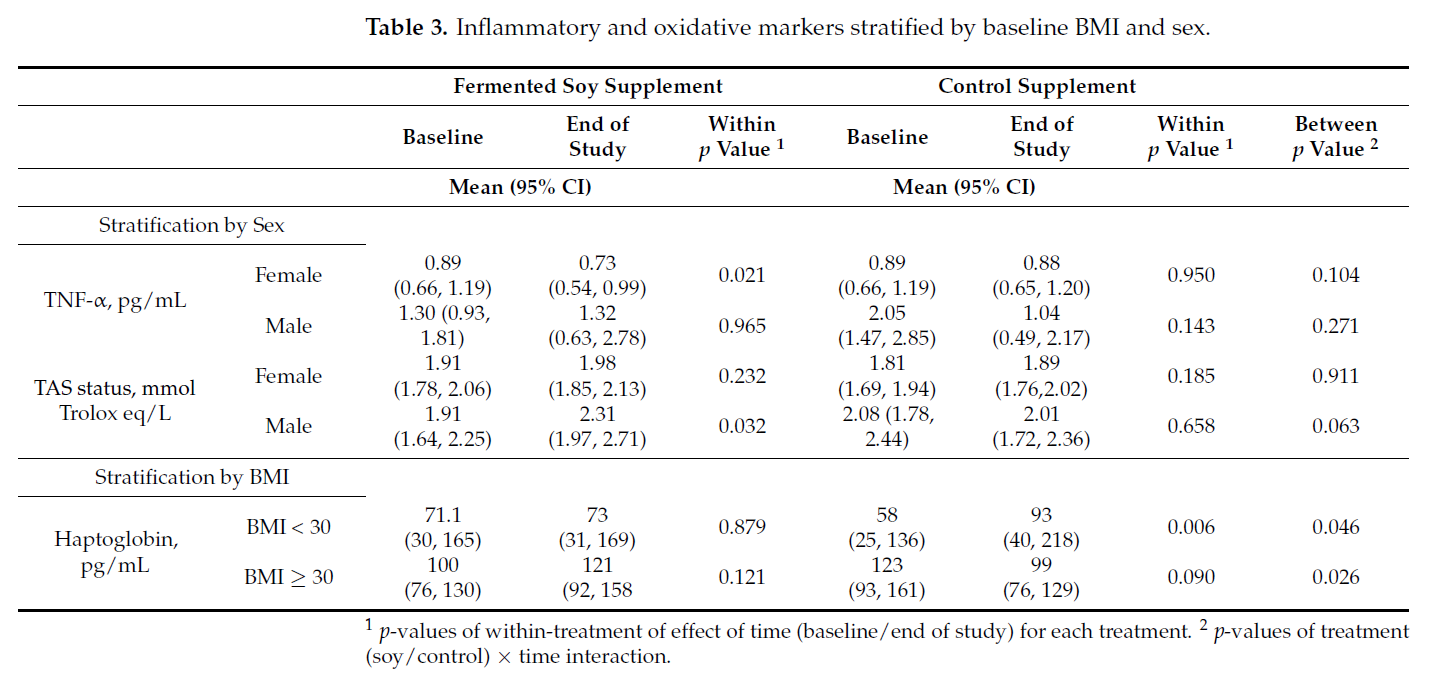
3.3. Stratification of Inflammatory and Oxidative Markers by Sex and Baseline BMI
To examine if the effect of treatment differs between the sexes, we further added sex
(male, female) and modeled a 3-way interaction of treatment × time × sex. Among all
the markers, the 3-way interaction was found to be significant only for TNF-α (p = 0.018)
and TAS (p = 0.032). Table 3 shows the outcomes of the sex-stratified analysis. TNF-α
levels in female participants were lower and TAS levels in male participants were higher
on the fermented soy intervention. Similarly, to examine if the effect of treatment is
different between obese (BMI ≥ 30) and non-obese (BMI < 30) at baseline, we modeled a
3-way interaction of treatment x time x BMI. The interaction was found to be significant
only for haptoglobin (p = 0.004). Haptoglobin levels were higher in those with BMI < 30
and lower in those with BMI ≥ 30 while on the control or brown rice intervention.
3.4. Canonical Correlation Analysis between Blood Lipids and Inflammation
To determine whether inflammatory, antioxidant, and blood lipid markers were associated in participants with CVD during the clinical trial, canonical correlation analysis
was used. Canonical correlation analysis is a multivariate analysis of associations between
two sets of variables, where each set consists of multiple related outcomes. It determines
linear combinations of variables (called canonical variates) from each set that maximize the
correlations among all possible linear combinations.
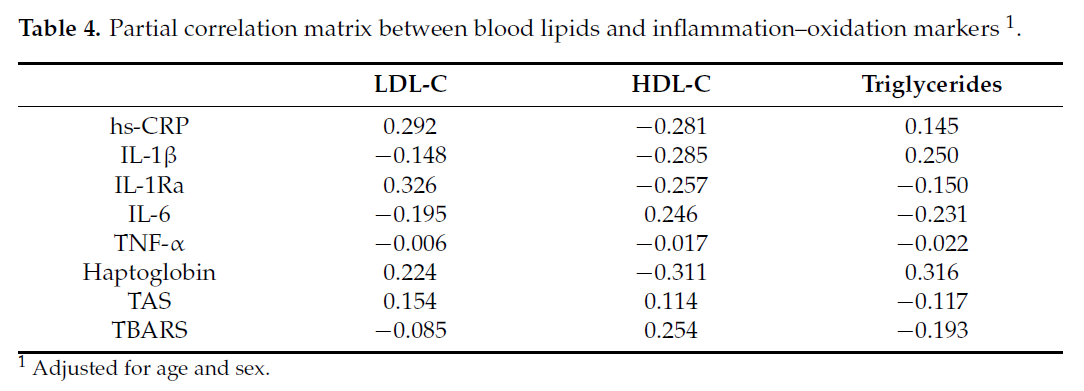
In the current study, inflammation was represented by a set of inflammatory and
oxidation markers composed of hsCRP, IL-1, IL1Ra, IL-6, TNF-α, haptoglobin, TAS, and
TBARS, all of which were log-transformed prior to analysis. Blood lipids were represented by LDL-C, HDL-C, and triglycerides. The loading coefficient of a canonical variate represents the contribution of each variable to the canonical variate. Table 4 shows the partial
correlation matrix that includes blood lipids and inflammation markers after adjusting for
age and sex. The partial correlation matrix is used to explore the inter-relationship between
blood lipids and inflammatory markers.
 The 1st and 2nd canonical variates between blood lipids and the inflammation–
The 1st and 2nd canonical variates between blood lipids and the inflammation–
oxidation markers are shown in Table 5. The loading coefficient of a canonical variate
represents the contribution of each variable to the canonical variate.
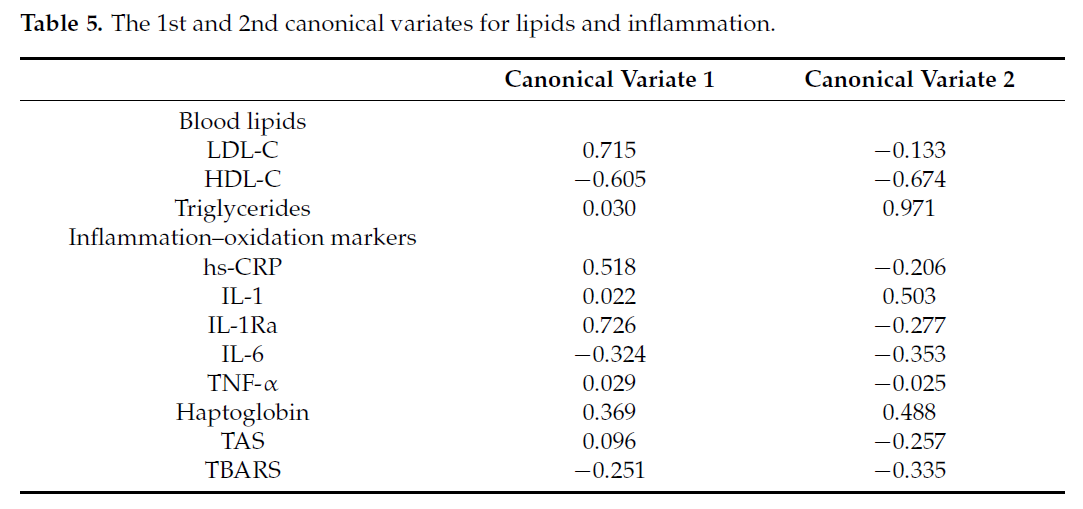 The 1st canonical variate for lipids was highly correlated with LDL-C (r = 0.715);
The 1st canonical variate for lipids was highly correlated with LDL-C (r = 0.715);
thus, this variate was most represented by LDL-C. The 2nd canonical variate is most
represented by triglycerides (r = 0.971) and both variates were negatively correlated with
HDL (r = -0.605 and -0.674, respectively). For inflammation, the 1st canonical variate was
represented by hsCRP and IL-1Ra, and the 2nd variate is represented by IL-1, haptoglobin,
and, to a lesser degree, IL-6, and TBARS. Helioplots showing correlations between canonical
variates and their components are shown in Figure 2.
The 1st correlation coefficient between the two sets of variables was r = 0.72 and
the corresponding pair of canonical variates explained 62% of the variability between the
biomarkers. The 2nd canonical correlation coefficient was r = 0.60 and the corresponding
pair of canonical variates explained 32% of the variability. This implies that hsCRP and
IL1-Ra appear to be positively associated with LDL-C and negatively with HDL-C and
IL-6 and haptoglobin appears to be positively correlated with triglycerides and negatively
with HDL-C.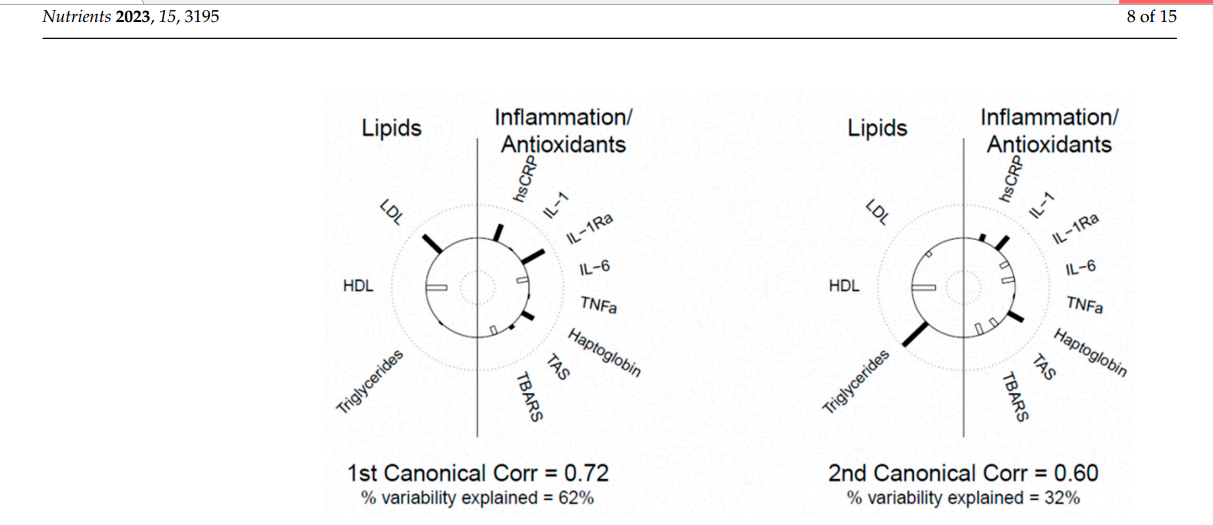
soy supplement dose. In a recent subgroup analysis of trials with isoflavone containing soy
products among women, serum IL-6 concentration increases occurred in studies where the
isoflavone doses were ≤87 mg per day, in studies where participants had chronic disease
risk factors and BMIs > 27 kg/m2, and in crossover studies [41]. The fermented soy supplement in the current study contained approximately 36 mg of isoflavones per daily dose and
participants were obese with a mean BMI of 32 kg/m2. On the other hand, the fermented
soy group showed an increase in TAS, a marker for the concentration of antioxidants in the
blood. Soy foods are known to have antioxidant effects [42] which may have an important
role in the recognized benefits of soy foods and their bioactive flavonoid components [6].
Since participants in the current study included a higher proportion of females and
obese individuals, stratification analysis was applied to determine whether the results of
the trial were confounded by sex or BMI. With regards to sex, TNF-α levels decreased
with the fermented soy supplement only in females, and although both males and females
on fermented soy showed increased TAS, a relatively greater increase occurred in males.
Gender differences due to soy foods are likely due to their content of isoflavones. It is
well-known that isoflavones as estrogens mimetics may exert pseudo-hormonal activity by
binding to estrogen receptors in females [43]. Although some clinical trials of soy foods
among women have shown reductions in TNF-α levels [12,17], findings from recent metaanalysis studies have been inconsistent [38,40]. One subgroup analysis of clinical trials
reported that inhibition of TNF-α generally occurred in studies utilizing lower doses of
isoflavones [31].
Stratification as a function of BMI showed an association with haptoglobin levels.
Haptoglobin is an acute phase α-glycoprotein secreted mainly by the liver whose major
biologic function is to strongly bind free hemoglobin, thus preventing renal and vascular
injury, loss of iron, and heme-initiated oxidation of proteins and membrane lipids [44,45].
In the absence of clearance, free hemoglobin can catalyze the formation of free radicals
that promote the oxidation of LDL-C [46]. In the present study, mean haptoglobin levels
were higher at baseline as a function of BMI, but only the control supplement intervention
showed treatment effects. The initial differences were also altered as a function of BMI
following the sprouted brown rice intervention with significant haptoglobin increases
in those with BMI < 30 and decreases in those with BMI ≥ 30. Overweight and obese
individuals are known to have elevated plasma haptoglobin and levels are significantly
reduced with weight loss [47]. Because of its role in the removal of oxidative species from
the circulation [48], both high and low levels of haptoglobin are indicative of a variety of
disorders with low levels associated with autoimmunity and increased levels occurring
in inflammation, diabetes, and cardiovascular disease [45]. However, few studies have
examined the effect of diet on this novel biomarker of systemic inflammation. Natural cocoa
consumption resulted in a significant decrease in haptoglobin in obese participants [49],
whereas the Mediterranean diet had no effect in a study of breast cancer survivors [50]. Our
findings suggest that the sprouted brown rice powder may not be an entirely neutral control
product and may contain bioactive compounds likely active in modulating biomarkers of
inflammation and oxidative stress [51].
As indicated above, we previously reported on the primary outcomes of the trial
and the effect of the fermented soy product in lowering total- and LDL-C [29]. Although
LDL-C remains an established causal risk factor for atherosclerosis, low-grade systemic
inflammation, and oxidative stress are risk enhancers and have crucial impacts on the
development and progression of coronary artery disease from endothelial dysfunction to
clinical syndromes [52,53], with hsCRP emerging as an independent predictor of CVD [54].
In the current analysis of the data, the association between blood lipids and inflammatory
markers in our otherwise healthy participant with risk factors for CVD was examined.
Since both blood lipids and the inflammatory markers are multidimensional and intercorre-lated with each other, it is challenging to directly evaluate the relationship between them.
Therefore, canonical correlational analysis, a multidimensional technique that can linearly
combine multiple factors into groups and analyze the correlation between the two groups’
variables, was applied [55]. For the inflammation biomarkers, the 1st canonical variate was
represented by hsCRP, IL-1Ra, and haptoglobin and these were positively correlated with
LDL-C (r = 0.715), whereas the 2nd canonical variate was represented by hsCRP, IL-1β,
and haptoglobin and correlated with triglycerides (r = 0.971). Both canonical variates
were negatively correlated with HDL (r = -0.605 and -0.674, respectively). Although few
nutrition and health studies apply canonical correlation to their data [56,57], these findings
support evidence of a close association between blood lipids and inflammation. Chronic
inflammation characterized by hs-CRP > 2 mg/L is linked to the development of CVD [58],
and a large secondary prevention trial of patients who have had a myocardial infarction
established that reducing inflammation with anti-cytokine therapy reduces the incidence of
CVD without changes in lipids [59]. To be effective, dietary interventions must concurrently
provide foods which modulate inflammation in addition to reducing atherogenic lipids.
In Asian countries, commercially fermented soy products such as Q-CAN and traditional fermented soybean foods, such as natto, tempeh, doenjang, and miso are commonly
consumed and are postulated to be more effective than unfermented soy foods in mitigating
lipids [60] and other atherogenic risks. Fermentation requires the involvement of microorganisms and produces a different kind of soy product often with improved nutritional
value and enhanced chemical and sensory qualities [61]. The fermentation process may
also inhibit some anti-nutrients found in soy and increase the digestibility of soy foods
and the bioavailability of their bioactive components [62]. Soybeans are one of the richest
sources of isoflavones and although native forms of soybean isoflavones are conjugated
with various sugars which reduces their bioavailability, fermentation has been reported
to increase the aglycone isoflavone content and increase isoflavone bioavailability [63,64].
Protein which constitutes approximately 40% of the soybean seed content may be degraded
into peptides by microbial proteases during fermentation [65]. Peptides produced from soy
fermentation are bioactive and exhibit various favorable effects, including antioxidant and
anti-inflammatory activity [32].
Although the functional properties associated with inflammation and oxidative stress
of fermented soy foods have been tested in numerous cell culture and animal studies [27,61,65], population studies and human intervention trials with fermented products
are limited. Recently a cross-sectional study in Japanese workers reported an inverse association between the consumption of fermented miso and soy sauce and plasma concentration
of the IL-6 cytokine in men, while dietary intake of non-fermented soy foods exhibited no
such correlation [66]. In another cross-sectional study in Japanese men, higher frequencies
of fermented soy product intake were associated with decreased arterial stiffness and
remained so even after adjusting for serum hs-CRP, a known biomarker for systemic inflammation [67]. In a randomized placebo-controlled clinical trial, a fermented soymilk product
containing Lactobacillus plantarum resulted in improved oxidative stress biomarkers in type
2 diabetic subjects [68]. A clinical trial with no control arm revealed that the fermented soy
beverage Q-CAN® Plus, similar to the one used in the current study, reduced serum levels
of platelet-derived growth factor in lean subjects and IL-1Ra and granulocyte-macrophage
colony-stimulating factor in obese subjects [30].
The exact mechanisms by which fermented soy foods exert their actions are unknown
and underlying mechanisms can vary due to the diversity of fermented foods and products
available and which are formulated with an array of microorganisms producing a multiplicity of bioactive constituents. Fermented soy benefits have been attributed to bioactive
peptides and amino acids, isoflavones both glycosylated and as aglycones, saponins, anthocyanins, and to atypical bacterial metabolites and breakdown products of sugars or
fatty acids as summarized in recent reviews [27,28,61,62,69]. Because the free flavonoid
content of fermented products is relatively higher and shows greater bioavailability than in
soybeans [70], many studies focused on the antioxidant and anti-inflammatory effects of soy flavonoids, particularly the isoflavones [69]. On a molecular level, extracts prepared from
fermented soy foods and isoflavones suppress activation of the transcription factor nuclear
factor- κB (NF-κB), a major effector in inflammatory and immune responses [71–73]. Fermented soy products have also been found to influence the Janus kinases/signal transducer
and activator of transcriptions (JAKs/STATs) and the mitogen-activated protein kinases
(MAPKs) pathways and to inhibit the expression of inflammatory cytokines [27,61,69]. Furthermore, constituents found in fermented foods such as bioactive peptides and saponins
are also anti-inflammatory and found to moderate NF-κB [32,74,75]. Mechanisms that
might explain the cardiometabolic benefits of consuming fermented soy foods may be
related to the observed changes in the oral and intestinal microbiome [76,77]. Hence, even
though it may not be possible to precisely identify the bioactive constituent in a fermented
soy food or its mechanism of action, the outcomes observed represent the synergistic effect
of all components of the product.
The strength of this study derives from the rigorous crossover design that allowed
participants to act as their own control and, thereby, minimize the influence of betweensubject variability when analyzing treatment effects. The study focused on participants
with at least two known CVD risk factors, and most were overweight or obese but had
inflammatory marker concentrations within normal ranges. However, the study had limitations. The number of subjects was determined based on the primary objective which
was the effect of the supplement on blood lipids and may have been underpowered for
assessing differences in inflammation and antioxidant outcomes. Several of our findings
were exploratory which limits their validity and p-values for the analyses were not corrected for multiple comparisons which increases the risk of type I error. Despite an open
recruitment policy, the participants were predominantly elderly and female which limits
the generalizability of the study. The odor and taste of the fermented soy supplement
were distinct; therefore, participant compliance and blinding may have been compromised.
Participants were advised to mix the study powders with water or milk, but some reported
difficulties in fully dissolving the powder and it is therefore possible that the full amount
was not consumed. More seriously, the germinated brown rice powder chosen as the
control supplement may not have been an entirely neutral product and may have had some
important anti-inflammatory and antioxidant properties [51,78].
In conclusion, the current crossover intervention trial showed a 12% increase in the
anti-inflammatory factor IL-1 Ra with the fermented soy product Q-CAN® compared to
the control powder whereas within-treatment effects of fermented soy showed 23% and 7%
increases in IL-6 and TAS, respectively. The first canonical correlation between inflammation
markers and blood lipids indicated hsCRP and IL-1Ra were positively associated with
LDL-C and negatively associated with HDL-C, which implies a close relationship between
blood lipids and inflammation. These findings indicate that fermented soy products may
impact blood lipids, antioxidants, and inflammatory factors and help inform future studies
on soy and inflammation.
Author Contributions: Conceptualization, E.H.H., J.S. and S.M.J.; methodology, E.H.H. and S.M.J.;
validation, A.K.; formal analysis, K.O.; investigation, S.M.J., A.K. and R.I.A.; data curation, S.M.J.,
A.K. and R.I.A.; writing—original draft preparation, S.M.J.; writing—review and editing, E.H.H.
and S.R.; visualization, K.O.; supervision, A.K.; project administration, A.K. and S.M.J.; funding
acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by Beso Biological Research Inc., Diamond Bar, CA, USA.
Institutional Review Board Statement: This study was conducted in accordance with the Declaration
of Helsinki and approved by the Institutional Review Board of Loma Linda University, IRB# 5180083
on 25 April 2018, and renewed every year hereafter.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: Detailed data related to this study are available on request from the
corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments: We are grateful to Eric Frongello and Beso Biological Research for providing the
fermented soy and the control intervention products for this study. We thank Claudia Aquilar for her
role as a phlebotomist and Rawiwan Sirirat for her role in managing the sample collection, aliquoting,
and storage.
Conflicts of Interest: The authors declare no conflict of interest.
References
1. Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-Nature’s Way to Efficiently Respond to All Types of
Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316.
[CrossRef] [PubMed]
2. Wu, L.; Shi, Y.; Kong, C.; Zhang, J.; Chen, S. Dietary Inflammatory Index and Its Association with the Prevalence of Coronary
Heart Disease among 45,306 US Adults. Nutrients 2022, 14, 4553. [CrossRef] [PubMed]
3. Bragagnolo, F.S.; Álvarez-Rivera, G.; Breitkreitz, M.C.; Ibáñez, E.; Cifuentes, A.; Funari, C.S. Metabolite Profiling of Soy ByProducts: A Comprehensive Approach. J. Agric. Food Chem. 2022, 70, 7321–7341. [CrossRef]
4. Lichtenstein, A.H.; Jalbert, S.M.; Adlercreutz, H.; Goldin, B.R.; Rasmussen, H.; Schaefer, E.J.; Ausman, L.M. Lipoprotein response
to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler.
Thromb. Vasc. Biol. 2002, 22, 1852–1858. [CrossRef] [PubMed]
5. Tikkanen, M.J.; Adlercreutz, H. Dietary soy-derived isoflavone phytoestrogens. Could they have a role in coronary heart disease
prevention? Biochem. Pharmacol. 2000, 60, 1–5. [CrossRef] [PubMed]
6. Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The soy isoflavone genistein reverses oxidative and
inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur. J. Pharmacol. 2011,
650, 694–702. [CrossRef]
7. Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003,
52, 341–346. [CrossRef]
8. Paradkar, P.N.; Blum, P.S.; Berhow, M.A.; Baumann, H.; Kuo, S.M. Dietary isoflavones suppress endotoxin-induced inflammatory
reaction in liver and intestine. Cancer Lett. 2004, 215, 21–28. [CrossRef]
9. Kao, T.H.; Wu, W.M.; Hung, C.F.; Wu, W.B.; Chen, B.H. Anti-inflammatory effects of isoflavone powder produced from soybean
cake. J. Agric. Food Chem. 2007, 55, 11068–11079. [CrossRef]
10. Wu, S.H.; Shu, X.O.; Chow, W.H.; Xiang, Y.B.; Zhang, X.; Li, H.L.; Cai, Q.; Ji, B.T.; Cai, H.; Rothman, N.; et al. Soy food intake and
circulating levels of inflammatory markers in Chinese women. J. Acad. Nutr. Diet. 2012, 112, 996–1004.e4. [CrossRef]
11. Nicastro, H.L.; Mondul, A.M.; Rohrmann, S.; Platz, E.A. Associations between urinary soy isoflavonoids and two inflammatory
markers in adults in the United States in 2005–2008. Cancer Causes Control CCC 2013, 24, 1185–1196. [CrossRef]
12. Huang, Y.; Cao, S.; Nagamani, M.; Anderson, K.E.; Grady, J.J.; Lu, L.J. Decreased circulating levels of tumor necrosis factor-alpha
in postmenopausal women during consumption of soy-containing isoflavones. J. Clin. Endocrinol. Metab. 2005, 90, 3956–3962.
[CrossRef] [PubMed]
13. Christie, D.R.; Grant, J.; Darnell, B.E.; Chapman, V.R.; Gastaldelli, A.; Sites, C.K. Metabolic effects of soy supplementation in
postmenopausal Caucasian and African American women: A randomized, placebo-controlled trial. Am. J. Obstet. Gynecol. 2010,
203, 153.e1–153.e9. [CrossRef]
14. Mangano, K.M.; Hutchins-Wiese, H.L.; Kenny, A.M.; Walsh, S.J.; Abourizk, R.H.; Bruno, R.S.; Lipcius, R.; Fall, P.; Kleppinger, A.;
Kenyon-Pesce, L.; et al. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: A randomized
controlled trial. Nutr. Res. 2013, 33, 1026–1033. [CrossRef] [PubMed]
15. Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with
nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [CrossRef]
16. Lebon, J.; Riesco, E.; Tessier, D.; Dionne, I.J. Additive effects of isoflavones and exercise training on inflammatory cytokines and
body composition in overweight and obese postmenopausal women: A randomized controlled trial. Menopause 2014, 21, 869–875.
[CrossRef] [PubMed]
17. Jalili, M.; Vahedi, H.; Poustchi, H.; Hekmatdoost, A. Soy isoflavones and cholecalciferol reduce inflammation, and gut permeability,
without any effect on antioxidant capacity in irritable bowel syndrome: A randomized clinical trial. Clin. Nutr. ESPEN 2019,
34, 50–54. [CrossRef] [PubMed]
18. Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin
resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018,
37, 1210–1215. [CrossRef]
19. Lesinski, G.B.; Reville, P.K.; Mace, T.A.; Young, G.S.; Ahn-Jarvis, J.; Thomas-Ahner, J.; Vodovotz, Y.; Ameen, Z.; Grainger, E.; Riedl,
K.; et al. Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory
cytokines and immunosuppressive cells. Cancer Prev. Res. 2015, 8, 1036–1044. [CrossRef]
20. Nadadur, M.; Stanczyk, F.Z.; Tseng, C.C.; Kim, L.; Wu, A.H. The Effect of Reduced Dietary Fat and Soy Supplementation on
Circulating Adipocytokines in Postmenopausal Women: A Randomized Controlled 2-Month Trial. Nutr. Cancer 2016, 68, 554–559.
[CrossRef]
21. Beavers, K.M.; Serra, M.C.; Beavers, D.P.; Cooke, M.B.; Willoughby, D.S. Soymilk supplementation does not alter plasma markers
of inflammation and oxidative stress in postmenopausal women. Nutr. Res. 2009, 29, 616–622. [CrossRef]
22. Greany, K.A.; Nettleton, J.A.; Wangen, K.E.; Thomas, W.; Kurzer, M.S. Consumption of isoflavone-rich soy protein does not alter
homocysteine or markers of inflammation in postmenopausal women. Eur. J. Clin. Nutr. 2008, 62, 1419–1425. [CrossRef]
23. Blum, A.; Lang, N.; Peleg, A.; Vigder, F.; Israeli, P.; Gumanovsky, M.; Lupovitz, S.; Elgazi, A.; Ben-Ami, M. Effects of oral soy
protein on markers of inflammation in postmenopausal women with mild hypercholesterolemia. Am. Heart J. 2003, 145, e7.
[CrossRef]
24. Jenkins, D.J.A.; Blanco Mejia, S.; Chiavaroli, L.; Viguiliouk, E.; Li, S.S.; Kendall, C.W.C.; Vuksan, V.; Sievenpiper, J.L. Cumulative
Meta-Analysis of the Soy Effect Over Time. J. Am. Heart Assoc. 2019, 8, e012458. [CrossRef] [PubMed]
25. Ryan-Borchers, T.A.; Park, J.S.; Chew, B.P.; McGuire, M.K.; Fournier, L.R.; Beerman, K.A. Soy isoflavones modulate immune
function in healthy postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 1118–1125. [CrossRef]
26. Reverri, E.J.; LaSalle, C.D.; Franke, A.A.; Steinberg, F.M. Soy provides modest benefits on endothelial function without affecting
inflammatory biomarkers in adults at cardiometabolic risk. Mol. Nutr. Food Res. 2015, 59, 323–333. [CrossRef] [PubMed]
27. Das, D.; Sarkar, S.; Borsingh Wann, S.; Kalita, J.; Manna, P. Current perspectives on the anti-inflammatory potential of fermented
soy foods. Food Res. Int. 2022, 152, 110922. [CrossRef] [PubMed]
28. do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential
Health Benefits: A Review. Microorganisms 2022, 10, 1606. [CrossRef]
29. Jung, S.M.; Haddad, E.H.; Kaur, A.; Sirirat, R.; Kim, A.Y.; Oda, K.; Rajaram, S.; Sabaté, J. A Non-Probiotic Fermented Soy Product
Reduces Total and LDL Cholesterol: A Randomized Controlled Crossover Trial. Nutrients 2021, 13, 535. [CrossRef]
30. Arumugam, S.; Dioletis, E.; Paiva, R.; Fields, M.R.; Weiss, T.R.; Secor, E.R.; Ali, A. Fermented Soy Beverage Q-CAN Plus
Consumption Improves Serum Cholesterol and Cytokines. J. Med. Food 2020, 23, 560–563. [CrossRef]
31. Asbaghi, O.; Yaghubi, E.; Nazarian, B.; Kelishadi, M.R.; Khadem, H.; Moodi, V.; Naeini, F.; Ghaedi, E. The effects of soy
supplementation on inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Cytokine
2020, 136, 155282. [CrossRef] [PubMed]
32. Kusumah, J.; Gonzalez de Mejia, E. Impact of soybean bioactive compounds as response to diet-induced chronic inflammation:
A systematic review. Food Res. Int. 2022, 162, 111928. [CrossRef] [PubMed]
33. Meier, C.A.; Bobbioni, E.; Gabay, C.; Assimacopoulos-Jeannet, F.; Golay, A.; Dayer, J.M. IL-1 receptor antagonist serum levels are
increased in human obesity: A possible link to the resistance to leptin? J. Clin. Endocrinol. Metab. 2002, 87, 1184–1188. [CrossRef]
34. Luotola, K. IL-1 Receptor Antagonist (IL-1Ra) Levels and Management of Metabolic Disorders. Nutrients 2022, 14, 3422. [CrossRef]
[PubMed]
35. Nicklin, M.J.; Hughes, D.E.; Barton, J.L.; Ure, J.M.; Duff, G.W. Arterial inflammation in mice lacking the interleukin 1 receptor
antagonist gene. J. Exp. Med. 2000, 191, 303–312. [CrossRef] [PubMed]
36. Uusitupa, M.; Hermansen, K.; Savolainen, M.J.; Schwab, U.; Kolehmainen, M.; Brader, L.; Mortensen, L.S.; Cloetens, L.; JohanssonPersson, A.; Onning, G.; et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation
markers in metabolic syndrome—A randomized study (SYSDIET). J. Intern. Med. 2013, 274, 52–66. [CrossRef]
37. Jenkins, D.J.; Kendall, C.W.; Connelly, P.W.; Jackson, C.J.; Parker, T.; Faulkner, D.; Vidgen, E. Effects of high- and low-isoflavone
(phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metab.
Clin. Exp. 2002, 51, 919–924. [CrossRef]
38. Rezazadegan, M.; Mirjalili, F.; Clark, C.C.T.; Rouhani, M.H. The effect of soya consumption on inflammatory biomarkers:
A systematic review and meta-analysis of clinical trials. Br. J. Nutr. 2021, 125, 780–791. [CrossRef]
39. Hariri, M.; Ghasemi, A.; Baradaran, H.R.; Mollanoroozy, E.; Gholami, A. Beneficial effect of soy isoflavones and soy isoflavones
plus soy protein on serum concentration of C-reactive protein among postmenopausal women: An updated systematic review
and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 59, 102715. [CrossRef]
40. Bajerska, J.; Łagowska, K.; Mori, M.; Reguła, J.; Skoczek-Rubi´nska, A.; Toda, T.; Mizuno, N.; Yamori, Y. A Meta-Analysis of
Randomized Controlled Trials of the Effects of Soy Intake on Inflammatory Markers in Postmenopausal Women. J. Nutr. 2022,
152, 5–15. [CrossRef]
41. Gholami, A.; Mollanoroozy, E.; Reza Baradaran, H.; Hariri, M. The efficacy of soy isoflavones combined with soy protein on
serum concentration of interleukin-6 and tumour necrosis factor-α among post-menopausal women? A systematic review and
meta-analysis of randomized controlled trials. Clin. Exp. Pharmacol. Physiol. 2022, 49, 10–24. [CrossRef]
42. Rizzo, G. The Antioxidant Role of Soy and Soy Foods in Human Health. Antioxidants 2020, 9, 635. [CrossRef] [PubMed]
43. Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol.
2010, 8, 89–98. [CrossRef] [PubMed]
44. Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, hemopexin, and related defense pathways-basic
science, clinical perspectives, and drug development. Front. Physiol. 2014, 5, 415. [CrossRef]
45. MacKellar, M.; Vigerust, D.J. Role of Haptoglobin in Health and Disease: A Focus on Diabetes. Clin. Diabetes Publ. Am. Diabetes
Assoc. 2016, 34, 148–157. [CrossRef] [PubMed]
46. di Masi, A.; De Simone, G.; Ciaccio, C.; D’Orso, S.; Coletta, M.; Ascenzi, P. Haptoglobin: From hemoglobin scavenging to human
health. Mol. Asp. Med. 2020, 73, 100851. [CrossRef]
47. Due, A.; Toubro, S.; Stender, S.; Skov, A.R.; Astrup, A. The effect of diets high in protein or carbohydrate on inflammatory markers
in overweight subjects. Diabetes Obes. Metab. 2005, 7, 223–229. [CrossRef] [PubMed]
48. Melamed-Frank, M.; Lache, O.; Enav, B.I.; Szafranek, T.; Levy, N.S.; Ricklis, R.M.; Levy, A.P. Structure-function analysis of the
antioxidant properties of haptoglobin. Blood 2001, 98, 3693–3698. [CrossRef]
49. McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Prado, E.A.; Best Sampson, J.N.; Vingren, J.L.; Hill, D.W. Natural cocoa consumption:
Potential to reduce atherogenic factors? J. Nutr. Biochem. 2015, 26, 626–632. [CrossRef]
50. Negrati, M.; Razza, C.; Biasini, C.; Di Nunzio, C.; Vancini, A.; Dall’Asta, M.; Lovotti, G.; Trevisi, E.; Rossi, F.; Cavanna, L.
Mediterranean Diet Affects Blood Circulating Lipid-Soluble Micronutrients and Inflammatory Biomarkers in a Cohort of Breast
Cancer Survivors: Results from the SETA Study. Nutrients 2021, 13, 3482. [CrossRef]
51. Ravichanthiran, K.; Ma, Z.F.; Zhang, H.; Cao, Y.; Wang, C.W.; Muhammad, S.; Aglago, E.K.; Zhang, Y.; Jin, Y.; Pan, B. Phytochemical
Profile of Brown Rice and Its Nutrigenomic Implications. Antioxidants 2018, 7, 71. [CrossRef] [PubMed]
52. Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [CrossRef]
53. Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022,
23, 2906. [CrossRef] [PubMed]
54. Danesh, J. Smoldering Arteries? Low-grade Inflammation and Coronary Heart Disease. JAMA 1999, 282, 2169–2171. [CrossRef]
[PubMed]
55. Laessig, R.E.; Duckett, E.J. Canonical correlation analysis: Potential for environmental health planning. Am. J. Public Health 1979,
69, 353–359. [CrossRef]
56. Galié, S.; García-Gavilán, J.; Papandreou, C.; Camacho-Barcía, L.; Arcelin, P.; Palau-Galindo, A.; Rabassa, A.; Bulló, M. Effects of
Mediterranean Diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a
crossover randomized clinical trial. Clin. Nutr. 2021, 40, 3798–3806. [CrossRef]
57. Liu, G.; Yang, S.; Liu, W.; Wang, S.; Tai, P.; Kou, F.; Jia, W.; Han, K.; Liu, M.; He, Y. Canonical Correlation Analysis on the
Association between Sleep Quality and Nutritional Status Among Centenarians in Hainan. Front. Public Health 2020, 8, 585207.
[CrossRef]
58. Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and
Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [CrossRef]
59. Everett, B.M.; MacFadyen, J.G.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Inhibition of Interleukin-1β and Reduction in
Atherothrombotic Cardiovascular Events in the CANTOS Trial. J. Am. Coll. Cardiol. 2020, 76, 1660–1670. [CrossRef]
60. Byun, M.S.; Yu, O.K.; Cha, Y.S.; Park, T.S. Korean traditional Chungkookjang improves body composition, lipid profiles and
atherogenic indices in overweight/obese subjects: A double-blind, randomized, crossover, placebo-controlled clinical trial.
Eur. J. Clin. Nutr. 2016, 70, 1116–1122. [CrossRef]
61. Cao, Z.H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019,
37, 223–238. [CrossRef] [PubMed]
62. Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [CrossRef]
[PubMed]
63. Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of isoflavones after ingestion of soy beverages in
healthy adults. J. Nutr. 2006, 136, 2291–2296. [CrossRef] [PubMed]
64. Champagne, C.P.; Tompkins, T.A.; Buckley, N.D.; Green-Johnson, J.M. Effect of fermentation by pure and mixed cultures of
Streptococcus thermophilus and Lactobacillus helveticus on isoflavone and B-vitamin content of a fermented soy beverage. Food
Microbiol. 2010, 27, 968–972. [CrossRef]
65. Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211.
[CrossRef]
66. Yang, X.; Nakamoto, M.; Shuto, E.; Hata, A.; Aki, N.; Shikama, Y.; Bando, Y.; Ichihara, T.; Minamigawa, T.; Kuwamura, Y.; et al.
Associations between intake of dietary fermented soy food and concentrations of inflammatory markers: A cross-sectional study
in Japanese workers. J. Med. Investig. 2018, 65, 74–80. [CrossRef]
67. Uemura, H.; Katsuura-Kamano, S.; Nakamoto, M.; Yamaguchi, M.; Fujioka, M.; Iwasaki, Y.; Arisawa, K. Inverse association
between soy food consumption, especially fermented soy products intake and soy isoflavone, and arterial stiffness in Japanese
men. Sci. Rep. 2018, 8, 9667. [CrossRef]
68. Hariri, M.; Salehi, R.; Feizi, A.; Mirlohi, M.; Ghiasvand, R.; Habibi, N. A randomized, double-blind, placebo-controlled, clinical
trial on probiotic soy milk and soy milk: Effects on epigenetics and oxidative stress in patients with type II diabetes. Genes Nutr.
2015, 10, 52. [CrossRef]
69. Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components,
mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [CrossRef]
70. Xu, L.; Du, B.; Xu, B. A systematic, comparative study on the beneficial health components and antioxidant activities of
commercially fermented soy products marketed in China. Food Chem. 2015, 174, 202–213. [CrossRef]
71. Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol.
2009, 1, a000034. [CrossRef] [PubMed]
72. Davis, J.N.; Kucuk, O.; Djuric, Z.; Sarkar, F.H. Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by
TNF-alpha in blood lymphocytes. Free Radic. Biol. Med. 2001, 30, 1293–1302. [CrossRef] [PubMed]
73. Ye, Y.; Zhou, J. The protective activity of natural flavonoids against osteoarthritis by targeting NF-κB signaling pathway. Front.
Endocrinol. 2023, 14, 1117489. [CrossRef] [PubMed]
74. Kim, I.S.; Yang, W.S.; Kim, C.H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [CrossRef]
[PubMed]
75. Luo, H.; Chen, J.; Su, C.; Zha, L. Advances in the Bioactivities of Phytochemical Saponins in the Prevention and Treatment of
Atherosclerosis. Nutrients 2022, 14, 4998. [CrossRef]
76. Dioletis, E.; Paiva, R.S.; Kaffe, E.; Secor, E.R.; Weiss, T.R.; Fields, M.R.; Ouyang, X.; Ali, A. The fermented soy beverage Q-CAN®
plus induces beneficial changes in the oral and intestinal microbiome. BMC Nutr. 2021, 7, 6. [CrossRef] [PubMed]
77. Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases.
Foods 2021, 10, 636. [CrossRef]
78. Mai, T.T.; Trang, T.T.; Hai, T.T. Effectiveness of germinated brown rice on metabolic syndrome: A randomized control trial in
Vietnam. AIMS Public Health 2020, 7, 33–43. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual
author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to
people or property resulting from any ideas, methods, instructions or products referred to in the content.
Previous
Categories
Recent posts
2025-06-03
