Prostate-Specific Antigen Modulatory Effect of a Fermented Soy Supplement for Patients with an Elevated Risk of Prostate Cancer: a Non-Randomized, Retrospective Observational Registration
2023-07-19
Prostate-Specific Antigen Modulatory Effect of a Fermented Soy Supplement for Patients with an Elevated Risk of Prostate Cancer: a Non-Randomized, Retrospective Observational Registration
Antonino Battaglia,a,b Gaëtan Devos,a,* Guy Boeckx,c Lieven Goeman,d Lorenzo Tosco,a Gert de Meerleer,e and Steven Joniaua
Author information Article notes Copyright and License information Disclaimer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7659407/
Abstract
Objective
To investigate the efficacy of a 6-month fermented soy supplement (equol-containing), measured by prostate-specific antigen (PSA) stabilization or PSA decrease from baseline (PSA modulatory effect) in men with an elevated risk of prostate cancer (PCa), with a WHO performance 0-2 and a follow-up of 12 months.
Methods
The patient population consisted of men with an elevated risk of PCa and a prior negative prostate biopsy within 1 year from starting therapy. Serum PSA values were recorded at inclusion (iPSA), at 6 months (1PSA), and optionally at 12 months (2PSA). Statistical analysis was carried out using the Wilcoxon rank sum test (p < 0.05).
Results
In total, 137 men used fermented soy for any prostatic reason. After inclusion criteria for an elevated risk of PCa and a prior negative prostate biopsy, we selected 58 patients. Among these, there was a significant PSA modulatory effect (iPSA-1PSA, p = 0.003). This modulatory effect was more strongly evident in the subgroup of patients with an elevated iPSA (≥ 4 ng/ml) (n = 33, iPSA-1PSA, p = 0.003, iPSA-2PSA, p = 0.002).
Conclusions
We demonstrated a significant PSA modulatory effect of a 6-month fermented soy supplement in men with an elevated risk of PCa and a prior negative prostate biopsy. This positive effect is currently being investigated in a prospective study. Further evaluation of the role of fermented soy supplements is warranted in a preventive and therapeutic setting of men at an elevated risk of PCa.
Key Words: Prostate, Prostate biopsy, Prostate cancer detection, Prostate specific antigen, Phytotherapeutics
Introduction
Prostate cancer (PCa) is the second most commonly diagnosed cancer in men worldwide with an estimated 1.1 million new cases in 2012 with almost 70% (759,000) of them occurring in more developed regions [1]. The incidence rate of PCa is much lower in Asian than in Western populations, especially in Eastern and South-Central Asia [1]. However, Asian migrants to the United States have a higher PCa incidence when they adopt a Western diet [2,3,4]. Similarly, PCa incidence and mortality has increased in Asian countries simultaneously with the westernization of their diet [5]. Therefore, environmental factors such as dietary habits have been presumed to play an important role in prostate carcinogenesis.
Soy foods, commonly consumed in Asian countries, are a rich source of isoflavones which possess anticancer activities. The mean isoflavone intake in Asian countries is approximately 50 mg daily, which is about 10 times higher than in Western countries [5,6]. Epidemiological studies showed that soy/isoflavones consumption in Asian populations is associated with a decrease in PCa risk [6]. Randomized controlled trials suggested there may be support for the epidemiological findings of a potential role for soy/soy isoflavones in PCa risk reduction. A good safety profile was also demonstrated for soy/soy isoflavones supplementation [7].
However, there is no clear understanding of the impact of soy/soy isoflavones on prostate-specific antigen (PSA) in men with, or at an identified risk of PCa [6,7,8]. During the past years we have conducted a small open-label study demonstrating a decrease or stabilization of PSA in men diagnosed with isolated high-grade prostatic intraepithelial neoplasia on biopsy in response to a 6-month dietary supplementation consisting of selenium, vitamin E, and soy isoflavones. It was shown in this study that a decrease in the PSA level while being on the supplement predicted a significantly lower risk of PCa in future biopsies [9].
The main isoflavones found in most soy foods are genistein, daidzein, and glycitein. Daidzein is converted to equol by the intestinal microflora of certain individuals [10]. Equol is 10 times more biologically potent than daidzein in delaying PCa growth [11]. The exact mechanism by which isoflavones and more specifically equol may prevent the development or progression of PCa remains unclear [12,13,14]. The efficacy of soy isoflavones appears to be a function of the individual's ability to produce equol [12,15]. Only 20-35% of Western adult populations host the intestinal bacteria that convert daidzein to equol [10]. Fermentation of soybeans results in extensive biotransformation of isoflavones with a significant increase in production of equol from daidzein. A possible strategy to increase the equol production is by changing the intestinal flora and changing non-equol producers to producers. A newly identified equol-producing bacterium was recently isolated from human feces of Japanese adults and further research is ongoing [16,17]. Nevertheless, the easiest way is to change the diet by adding nutrients such as fermented soy (equol) in the form of a supplement. PraëCell® is a unique and patented supplement based on specific soybeans (ZhenHua) from the Chinese highlands fermented by a unique strain of bacteria [18]. Consumption of dietary supplements is an interesting approach in PCa as non-invasive active surveillance has increased in popularity as a strategy for reducing potential overtreatment of this slow-growing but potentially fatal disease.
Considering all of the above and starting from the experience of our last study [9], we hypothesized that the intake of a fermented soy supplement by patients with an elevated PCa risk might modulate PSA and help to select out the population at real increased risk of having occult PCa. Such preventive strategy has many advantages, as it is easy to use, non-invasive, non-toxic (and well tolerated), and not expensive. This retrospective study investigated the effect of a 6-month dietary supplement with fermented soy as the main component on the PSA level in 58 men with an elevated risk of PCa and a prior negative prostate biopsy.
Materials and Methods
Subjects
A group of 137 patients received fermented soy for any prostatic disease between November 4th, 2013 and April 21st, 2016. We included in the analysis only patients with an elevated risk of PCa and with a negative prostate biopsy performed within 12 months from starting treatment. An elevated risk of PCa was defined by any one of the following criteria: PSA > 4 ng/ml, PSA density > 0.15 ng/ml/ml, PSA velocity > 0.75 ng/ml/y, suspect lesion at digital rectal examination (DRE), suspect lesion at transrectal ultrasound (TRUS)/magnetic resonance imaging (MRI), and family history of PCa. Patients with a lower performance status and those with a known history of soy allergy were excluded. Patients were included when they had a follow-up of at least 6 months [median 11 months, interquartile range (IQR): 7.5-17 months]. Data were acquired at 6, 12, and 18 months.
Fermented Soy Supplement
All patients were given PraëCell® (Health, Science & Nutrition, Antwerp, Belgium), 1 capsule per day for at least 6 months. Praëcell® is a commercially available, dietary supplement therapy consisting of fermented soy (ZhenHua) (60 mg), isoflavones (10 mg), vitamin C (90 mg), beta-glucan (105 mg), lycopene (3 mg), vitamin D (5 µg), zinc (5 mg), and selenium (27.5 µg) per capsule.
Study Design and Endpoint
This paper presents the results of a 6-month, non-randomized, open-label, retrospective registration study investigating the effect of a fermented soy supplement in men with an elevated risk of PCa and a prior negative prostate biopsy (Fig. (Fig.1).1). The endpoint of this trial was the efficacy of a fermented soy supplement, measured by PSA stabilization or PSA decrease from the baseline (PSA modulatory effect).
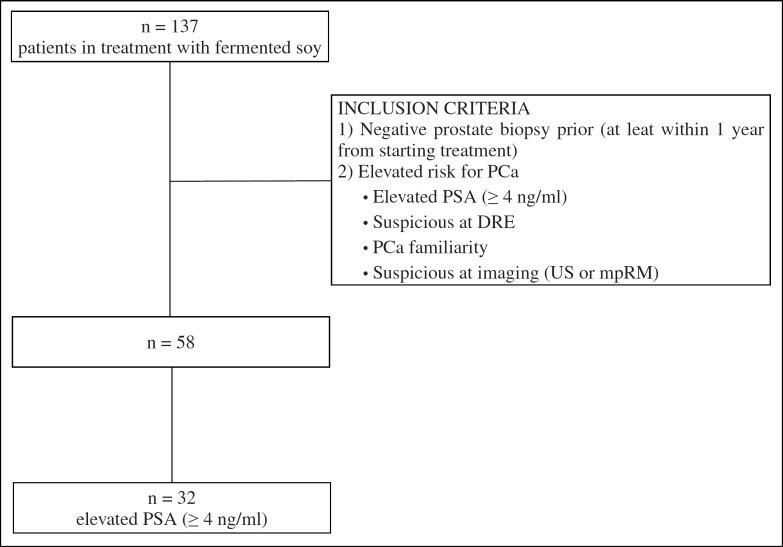
Retrospective registration study investigating the effect of a fermented soy supplement in men with an elevated risk of prostate cancer and a prior negative prostate biopsy. Inclusion criteria are provided in the figure. In total, 58 patients were included of which 32 had an elevated PSA > 4 ng/ml.
Measurements
At the baseline, at 6 months, and optionally at 12 months, blood samples were obtained for PSA measurement. A DRE was performed at baseline and all follow-up visits. TRUS or multipara-metric MRI and an estimation of prostate volume were performed at baseline and were optional at follow-up visits. TRUS-guided needle biopsy was performed within 12 months from starting treatment.
Statistical Analysis
Principles of descriptive statistics were used to report patient characteristics at the baseline, technical imaging was used and histological specimens after biopsy with relative percentages and median with IQR according to the values. Statistical analysis were carried out using the Wilcoxon rank sum test to evaluate the assumption that fermented soy changes the serum PSA with statistical significance (p < 0.05). Graphics with boxplots illustrate the results in changing PSA. Data were analyzed using the statistical software R version 3.3.2.
Results
In total, 137 men used fermented soy for any prostatic reason. After inclusion criteria for an elevated risk of PCa and a prior negative prostate biopsy, we selected 58 patients. Their median age was 68 years (IQR: 58-73 years). Median follow-up was 11 months. Descriptive statistics are presented in Table Table11.
Table 1
Descriptive statistics for patients treated with a fermented soy supplement between November 2013 and April 2016 at a single center
Values | |
|---|---|
Patient characteristics | |
Total, n | 58 |
Median age (IQR), years | 68 (58–73) |
Median prostate volume (IQR), ml | 20 (15–35) |
Positive familiarity, n (%) | 4 (6.9) |
DRE | |
Normal, n (%) | 24 (41.4) |
Hardness, n (%) | 4 (6.9) |
Benign prostatic hyperplasia, n (%) | 21 (36.2) |
Prostatitis, n (%) | 2 (3.4) |
Imaging | |
Ultrasound suspicious area or multiparametric MRI, n (%) | 10 (17.2) |
Normal, n (%) | 9 (15.5) |
PCa, n (%) | 3 (5.2) |
Doubt, n (%) | 7 (12.1) |
Prostatitis, n (%) | 1 (1.7) |
PIRADS 4–5, n (%) | 4 (6.9) |
Median follow-up (IQR), months | 11 (7.5–17) |
PIRADS = Prostate Imaging Reporting and Data System.
Table Table22 shows the statistical significance (Wilcoxon rank sum test) of the PSA measured at the first control (iPSA-1PSA) and PSA measured at the second control (iPSA-2PSA) after fermented soy supplementation compared with the initial PSA at inclusion. For the subgroup of patients with an elevated PSA, the statistical significance of the 2PSA compared to the 1PSA (1PSA-2PSA) is also reported.
Table 2
Wilcoxon rank sum test for analyzing the PSA modulatory effect of a 6-month fermented soy supplement in patients with elevated risk of PCa (p < 0.05)
n | p | |
|---|---|---|
Number of PCa, prior negative prostate biopsy | 58 | |
iPSA–1PSA | 0.003 | |
iPSA–2PSA | 0.125 | |
Elevated PSA (≥ 4 ng/ml), prior negative prostate biopsy | 33 | |
iPSA–1PSA | 0.003 | |
iPSA–2PSA | 0.002 | |
1PSA–2PSA | 0.52 |
The 1PSA modulatory effect compared with the initial PSA values at inclusion reached statistical significance in the study group of patients with an elevated risk of PCa and a prior negative prostate biopsy (n = 58) (iP-SA-1PSA, p = 0.003) (Fig. (Fig.2).2). The 1PSA and 2PSA modulatory effect compared with the initial PSA values at inclusion reached statistical significance in the subgroup of patients with an elevated PSA (n = 33) (iPSA-1PSA, p = 0.003, iPSA-2PSA, p = 0.002) (Fig. (Fig.3).3). The PSA modulatory effect of the fermented soy supplement was more strongly evident in the subgroup of patients with an elevated iPSA.
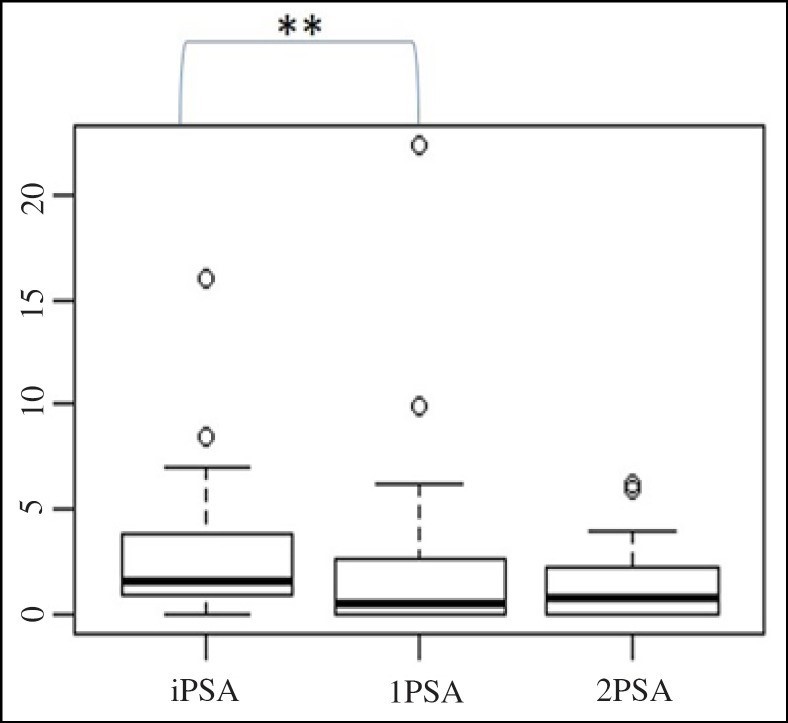
PSA modulatory effect of a fermented soy supplement in patients with an elevated risk of PCa and a prior negative prostate biopsy (n = 58). **The difference between iPSA and 1PSA was statistically significant (iPSA-1PSA, p = 0.003).
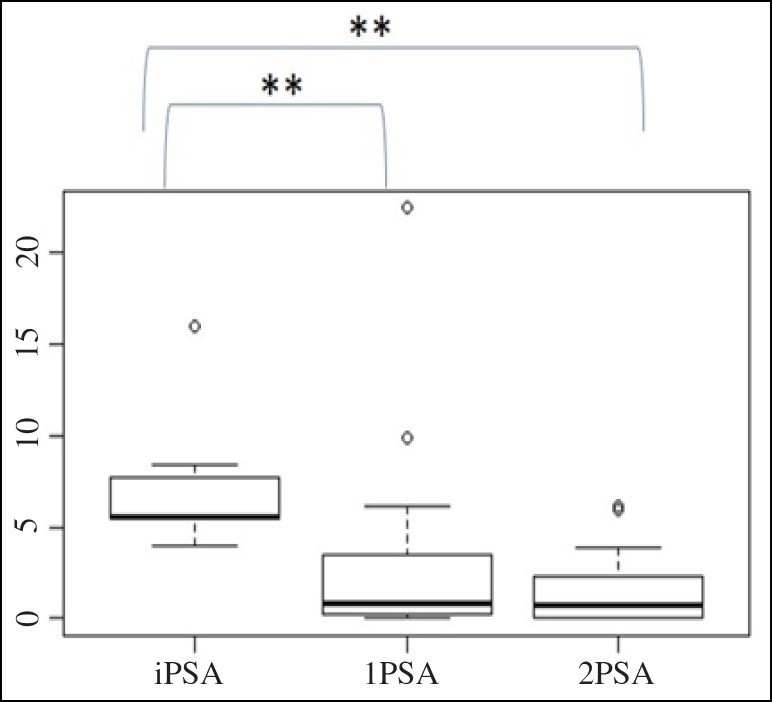
PSA modulatory effect of a fermented soy supplement in patients with an elevated risk of PCa, a prior negative prostate biopsy and elevated PSA (≥ 4 ng/ml) (n=32). **The difference between iPSA-1PSA and iPSA-2PSA was statistically significant (iPSA-1PSA, p = 0.003; iPSA-2PSA, p=0.002).
Figure Figure44 and and55 present the decrease of the PSA value during consumption of the fermented soy supplement in the study group (n = 58) and in the subgroup (n = 33), respectively.
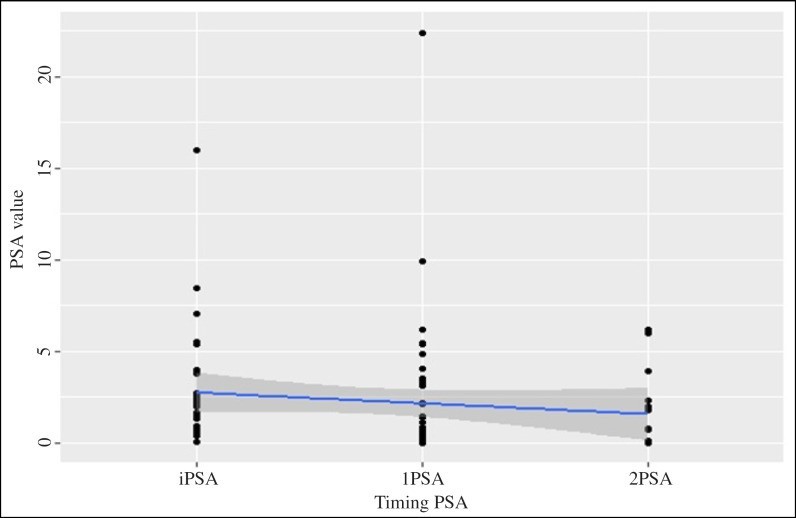
Decrease of the PSA value during consumption of the fermented soy supplement in patients with an elevated risk of PCa and a prior negative prostate biopsy (n = 58).
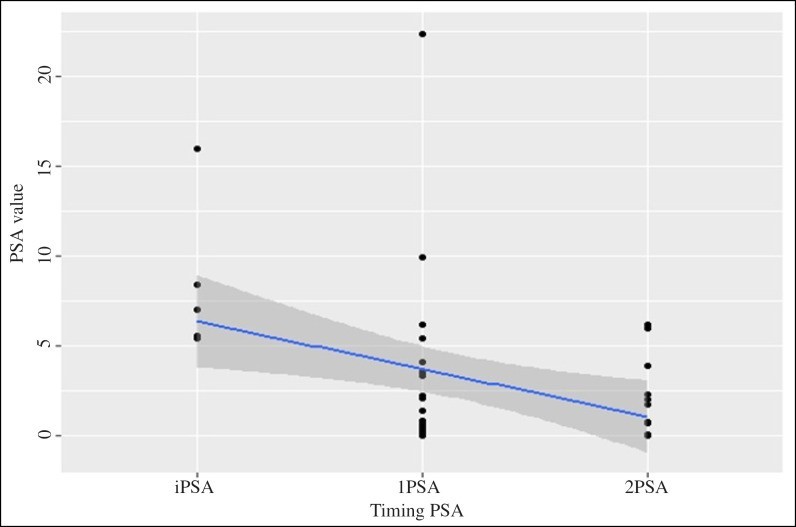
Decrease of the PSA value during consumption of the fermented soy supplement in patients with an elevated risk of PCa, a prior negative prostate biopsy and elevated PSA (≥ 4 ng/ml) (n = 32).
Discussion
Our study has shown that in men with an elevated PCa risk and a prior negative prostate biopsy, the PSA level remained stable or decreased from the baseline while being on a fermented soy supplement for 6 months. This finding is interesting, because several studies had not been able to show a reduction in PSA while on a soy diet or soy isoflavones supplement. Shortcomings of many studies published to date were small patient numbers, lack of randomization, short-term isoflavone administration, possibly insufficient doses, the variety of forms in which isoflavones were administered, and the heterogeneity of dosage regimens and study populations.
Eight randomized controlled trials (RCT) were conducted to evaluate the efficacy and the safety of soy/soy isoflavones in men with histologically confirmed PCa (6 RCTs) or with a clinically identified risk of PCa (2 RCTs) [7]. A meta-analysis of the 2 RCTs investigating the development of PCa in men with a clinically identified risk [19,20] showed a statistically significant reduction in PCa diagnosis after the administration of soy/soy isoflavones (relative risk = 0.49, 95% confidence interval 0.26-0.95) [7]. An individual Japanese RCT of 158 men with serum PSA levels of 2.5-10.0 ng/ml, and a single, negative prostate biopsy within 12 months prior to enrolment, showed a significant lower incidence of PCa in the isoflavones group (60 mg/d) compared to the placebo group for 53 patients aged ≥ 65 years (28 vs. 57.1%, p = 0.031). No significant differences in PSA levels within or between groups were observed [19]. The other RCT included 58 patients with high risk for future PCa (high-grade prostatic intraepithelial neoplasia, atypical small acinar proliferation) or low-grade PCa. Less PCa was detected after 6 months of a daily consumption of 40 mg soy protein (107 mg isoflavones). PSA levels did not differ among the groups at 3 and 6 months [20].
Several RCTs and small non-randomized studies investigating soy protein/soy isoflavones supplements that included PSA endpoints were conducted in healthy men, men with PCa undergoing active surveillance, men with untreated localized PCa, and men with an elevated PSA after radical prostatectomy or radiation. A meta-analysis of 7 RCTs [7] that included PSA endpoints showed no statistically significant effect on PSA levels in men with PCa [21,22,23,24,25], or who were at risk of PCa [19,20]. However, in two individual RCTs, the decrease in PSA was significant [25] or approached significance [21]. In the first RCT, the PSA and the free/total PSA ratio were favorably influenced in men consuming bread high in heat-treated soy grits (change in PSA level; −12.7% for soy grits vs. 40% for wheat, p = 0.02) [25]. In the second RCT, daily consumption of 30 mg genistein for 3-6 weeks prior to prostatectomy reduced serum PSA levels in patients with localized PCa. PSA decreased by 7.8% in the genistein arm and increased by 4.4% in the placebo arm (p = 0.051) [21]. In addition, Kumar et al. [23] showed that in a group of early PCa patients (Gleason score 6 or less), the serum total PSA decreased or was unchanged in 69% of the patients treated with isoflavones (60 mg) compared to 55% in the placebo group. In 19% of patients receiving soy isoflavones, the serum total PSA was reduced by ≥ 2 points during the intervention period. Moreover, Hussain et al. [26] showed that although soy isoflavones supplements did not provoke sustained decreases in PSA, stabilization of the PSA occurred in 83% of hormone-sensitive PCa patients and 35% of hormone-refractory PCa patients. In a more recent RCT, daily consumption of a 20 mg soy protein isolate supplement for 24 months did not reduce biochemical recurrence of PCa after radical prostatectomy [27].
An important difference between our study and previous studies was the use of fermented soy (equol) as the main substance of our dietary supplement in patients with an elevated risk of PCa. Equol which is metabolized from daidzein by certain human intestinal bacteria, is biologically more active in delaying PCa growth than any other isoflavone aglycone [11]. A case-control study showed that the percentage of equol producers in patients with PCa was significantly lower than in healthy controls (30.3 vs. 49.5%, p = 0.013) [28]. Our analysis demonstrated several important findings. In the group of patients with an elevated risk of PCa and a prior negative prostate biopsy, the PSA modulatory effect reached significance, at the first control after starting treatment with the fermented soy supplement. The most remarkable findings were observed in the subgroup of patients with an elevated PSA (≥ 4 ng/ml). In this subgroup, the PSA modulatory effect at the first and second control after starting treatment with the fermented soy supplement reached statistical significance, compared with the initial PSA values at inclusion.
The current study is not devoid of limitations. First, our findings must be interpreted with all the limitations related to retrospective studies. Second, our conclusions are based on group analyses with small sample sizes.
Further well-designed studies are warranted to determine the positive effect of the fermented soy supplement on PSA levels and PCa risk. A prospective study is designed by our department to fully investigate the effectiveness of fermented soy supplement intake, measured by the PSA modulatory effect, in patients with an elevated risk of PCa and prior negative prostate biopsies. The protocol of this prospective study was approved by the Ethics Committee of the University Hospital KU Leuven on March the 3rd, 2017. If this prospective study confirms the PSA-modulatory effect of a fermented soy supplement (stabilize or decrease PSA) in patients with an elevated PCa risk, prescribing such supplement can help the urologist to select out the patients at real in-creased risk of having occult PCa. When the PSA level increases from the baseline during fermented soy consumption, additional prostate biopsies remain necessary and adequate treatment may be needed.
Conclusions
In this study, we demonstrated a significant PSA modulatory effect of a 6-month intake of a fermented soy supplement in men with an elevated risk of PCa and a prior negative prostate biopsy. This modulatory effect was more strongly evident in the subgroup of patients with an elevated PSA (≥ 4 ng/ml). The positive effect of a fermented soy supplement is currently being investigated in a prospective study. Further evaluation of the role of fermented soy supplements is warranted in the preventive and therapeutic setting of men with an elevated risk of PCa.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. [PubMed] [Google Scholar]
2. Marks LS, Kojima M, Demarzo A, Heber D, Bostwick DG, Qian J, Dorey FJ, Veltri RW, Mohler JL, Partin AW. Prostate cancer in native Japanese and Japanese-American men: effects of dietary differences on prostatic tissue. Urology. 2004;64:765–771. [PubMed] [Google Scholar]
3. Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. [PubMed] [Google Scholar]
4. Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012;31:421–429. [PMC free article] [PubMed] [Google Scholar]
5. Gardner CD, Oelrich B, Liu JP, Feldman D, Franke AA, Brooks JD. Prostatic soy isofla-vone concentrations exceed serum levels after dietary supplementation. Prostate. 2009;69:719–726. [PMC free article] [PubMed] [Google Scholar]
6. Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–1163. [PubMed] [Google Scholar]
7. van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113:E119–E130. [PubMed] [Google Scholar]
8. Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. [PubMed] [Google Scholar]
9. Joniau S, Goeman L, Roskams T, Lerut E, Oyen R, Van Poppel H. Effect of nutritional supplement challenge in patients with isolated high-grade prostatic intraepithelial neo-plasia. Urology. 2007;69:1102–1106. [PubMed] [Google Scholar]
10. Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. [PubMed] [Google Scholar]
11. Hedlund TE, Johannes WU, Miller GJ. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2003;54:68–78. [PubMed] [Google Scholar]
12. Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132. [PMC free article] [PubMed] [Google Scholar]
13. Itsumi M, Shiota M, Takeuchi A, Kashiwagi E, Inokuchi J, Tatsugami K, Kajioka S, Uchiumi T, Naito S, Eto M, Yokomizo A. Equol inhibits prostate cancer growth through degradation of androgen receptor by S-phase kinase-associated protein 2. Cancer Sci. 2016;107:1022–1028. [PMC free article] [PubMed] [Google Scholar]
14. Lu Z, Zhou R, Kong Y, Wang J, Xia W, Guo J, Liu J, Sun H, Liu K, Yang J, Mi M, Xu H. S-equol, a secondary metabolite of natural anticancer isoflavone daidzein, inhibits prostate cancer growth in vitro and in vivo, though activating the Akt/FOXO3a pathway. Curr Cancer Drug Targets. 2016;16:455–465. [PubMed] [Google Scholar]
15. Akaza H. Prostate cancer chemoprevention by soy isoflavones: role of intestinal bacteria as the “second human genome” Cancer Sci. 2012;103:969–975. [PMC free article] [PubMed] [Google Scholar]
16. Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch Microbiol. 2010;192:279–287. [PubMed] [Google Scholar]
17. Nagata Y, Sugiyama Y, Fukuta F, Takayanagi A, Masumori N, Tsukamoto T, Akasaka H, Ohnishi H, Saitoh S, Miura T, Moriyama K, Tsuji H, Akaza H, Mori M. Relationship of serum levels and dietary intake of isoflavone, and the novel bacterium Slackia sp. strain NATTS with the risk of prostate cancer: a case-control study among Japanese men. Int Urol Nephrol. 2016;48:1453–1460. [PubMed] [Google Scholar]
18. Yang Z, Liu S, Chen X, Chen H, Huang M, Zheng J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000;60:505–509. [PubMed] [Google Scholar]
19. Miyanaga N, Akaza H, Hinotsu S, Fujioka T, Naito S, Namiki M, Takahashi S, Hirao Y, Horie S, Tsukamoto T, Mori M, Tsuji H. Prostate cancer chemoprevention study: an investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012;103:125–130. [PubMed] [Google Scholar]
20. Hamilton-Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer. 2008;60:7–13. [PubMed] [Google Scholar]
21. Lazarevic B, Boezelijn G, Diep LM, Kvern-rod K, Ogren O, Ramberg H, Moen A, Wessel N, Berg RE, Egge-Jacobsen W, Hammar-strom C, Svindland A, Kucuk O, Saatcioglu F, Taskèn KA, Karlsen SJ. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr Cancer. 2011;63:889–898. [PubMed] [Google Scholar]
22. deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62:1036–1043. [PMC free article] [PubMed] [Google Scholar]
23. Kumar NB, Cantor A, Allen K, Riccardi D, Besterman-Dahan K, Seigne J, Helal M, Salup R, Pow-Sang J. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59:141–147. [PubMed] [Google Scholar]
24. Kumar NB, Kang L, Pow-Sang J, Xu P, Allen K, Riccardi D, Besterman-Dahan K, Krischer JP. Results of a randomized phase I dose-finding trial of several doses of isoflavones in men with localized prostate cancer: administration prior to radical prostatectomy. J Soc Integr Oncol. 2010;8:3–13. [PMC free article] [PubMed] [Google Scholar]
25. Dalais FS, Meliala A, Wattanapenpaiboon N, Frydenberg M, Suter DA, Thomson WK, Wahlqvist ML. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64:510–515. [PubMed] [Google Scholar]
26. Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–117. [PubMed] [Google Scholar]
27. Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E, Melamed J, Kong MX, Macias V, Kajdacsy-Balla A, Lumey LH, Xie H, Gao W, Walden P, Lepor H, Taneja SS, Randolph C, Schlicht MJ, Meserve-Watanabe H, Deaton RJ, Davies JA. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. 2013;310:170–178. [PMC free article] [PubMed] [Google Scholar]
28. Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, Fujioka T, Mori M, Kim WJ, Song JM, Pantuck AJ. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–89. [PubMed] [Google Scholar]
Articles from Current Urology are provided here courtesy of Wolters Kluwer Health
Previous
Next
Categories
Recent posts
2025-06-03
